December 2012 – Englewood, New Jersey
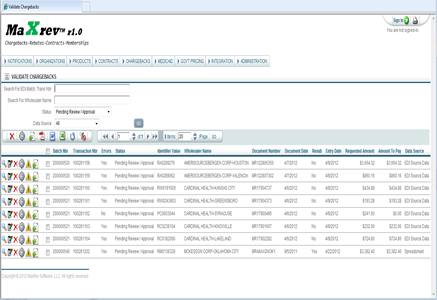
MPS Associates LLC is proud to announce that we have been chosen to assist Englewood Lab, a leading New Jersey based developer and contract manufacturer of cosmetic and OTC consumer products with the computer system validation of their enterprise resource planning system. The client will utilize MPS’ proprietary MFG-Validate toolset with MPS assuming responsibility for the development of all required validation documents including SVMP/IQ/OQ/PQ protocols. Contact Mike Malinchak to learn how MPS can fullfill your Company’s computer system validation requirements.